Chlorine Dioxide Analyser – DioSense
 Online, continuous Chlorine Dioxide Analysers and Controllers make up the DioSense range which utilises the very latest and best chlorine dioxide sensors available in the world today. The DioSense chlorine dioxide sensors are membraned devices which are insensitive to chlorine, use no reagents, are extremely stable, and have reduced maintenance and reduced whole life costs.
Online, continuous Chlorine Dioxide Analysers and Controllers make up the DioSense range which utilises the very latest and best chlorine dioxide sensors available in the world today. The DioSense chlorine dioxide sensors are membraned devices which are insensitive to chlorine, use no reagents, are extremely stable, and have reduced maintenance and reduced whole life costs.
The DioSense chlorine dioxide sensors which give a dissolved chlorine dioxide measurement in ppm or mg/l and their associated flow cells are available with different chlorine dioxide controllers giving you the same great performance with different communication, display, and control options.
The DioSense Residual Chlorine Dioxide Analyser is used in many applications requiring chlorine dioxide analysers, either simply for chlorine dioxide monitoring or for residual chlorine dioxide dosing control.
The membraned amperometric chlorine dioxide sensor used in the DioSense chlorine dioxide analyser is a two electrode sensor which operates at an elevated applied potential which in turn eliminates zero drift. Its unique design means that no reagents or buffers are required at all and calibration is a simple one point (no zero required) operation.
In addition to the state of the art amperometric chlorine dioxide sensors, the DioSense range of chlorine dioxide controllers has all the functionality that you need. Choose from the CRONOS® or CRIUS®4.0 controller to give you the highest quality chlorine dioxide analyser, with all the functionality you need, at the lowest price possible. Its modular design means that you pay for everything that you need, and nothing you don’t, without sacrificing the quality of measurement.
The DioSense chlorine dioxide analyser is used in many applications from hospital water treatment to agricultural produce washing often as part of a chlorine dioxide dosing control regime. Capabilities including remote access via the mobile phone network and full ClO2 dosing control, marking the DioSense as the chlorine dioxide analysers of choice for many ClO2 suppliers.
Pi’s chlorine dioxide analysers are designed to have reduced maintenance, reduced calibration and reduced spares requirements. The DioSense ClO2 analysers are undeniably the most cost effective ClO2 analysers available.
According to the EU Directive 98/83/EC, the operators of waterworks must ensure that the levels of harmful chlorite (ClO2–) in drinking water remain below the specified limit of 0.2 mg/l. The monitoring of chlorite at the same time as chlorine dioxide is also a European requirement.
Pi is able to provide both chlorine dioxide and chlorite sensors which can be controlled from a single chlorine dioxide analyser. In most situations the ClO2 analyser will be able to control the dosing of ClO2 by adjusting flow rates, pump rates, or valve positions automatically to maintain the residual chlorine dioxide set-point. Automatic dosing can significantly reduce reagent costs, and increase the level of control over harmful chlorite levels using the DioSense chlorine dioxide analysers.
Chlorine dioxide monitors are often used to monitor the residual ClO2 in difficult ‘dirty’ applications such as in hospitals or the food industry. In these cases, in order to maintain an appropriate chlorine dioxide residual it is necessary to automatically clean the sensor using the AutoFlush.
If you think your application could need the AutoFlush, please contact us and discuss it with our experienced technical sales people.
 Amperometric sensors – continuous online chlorine dioxide ppm measurement
Amperometric sensors – continuous online chlorine dioxide ppm measurement- No chemical reagents – lower cost of ownership
- Stable and reliable – excellent process control
- Suitable for all potable and process waters
- Up to 6 months between maintenance
- No interference from residual chlorine
- Tolerant of water containing detergents
The DioSense chlorine dioxide measurement sensors and flow cells are available with different controllers giving you the same great performance with different communication, display, and chlorine dioxide dosing control options.
The two controllers offer different options but both offer the same great user interface and Pi’s great quality.
- Chlorine Dioxide Dosing Control
- Remote Sites – remote access available
- Cooling Towers
- Food Preparation
- Hospitals
- Food & Drink Industry
- Paper Mills
Anywhere you have a requirement to measure and/or control residual ClO2 is a suitable application for the DioSense. The DioSense chlorine dioxide monitor range is particularly suited to working in sites where reliability and ease of use are most important.
Book a ClO2 Meter Demonstration
Why not click here and ask for a demonstration of all that our chlorine dioxide analysers can do for you?
As described elsewhere, the DioSense online chlorine dioxide monitors can come equipped to automatically clean themselves at user defined intervals. The AutoFlush is particularly useful in food preparation, pulp and paper, and many applications where there is likely to be a build up of solids in the sample. The DioSense range of chlorine dioxide monitors are particularly resistant to tensides and are therefore particularly useful in food washing applications.
The whole range of DioSense Residual Chlorine Dioxide Monitors and Controllers can be fitted with additional sensors such as chlorite or pH. Contact Pi for more details.
- Chlorine dioxide is created on site, eliminating the need to store the chlorine and/or the transportation.
- Chlorine dioxide functions through an oxidative reaction rather than chlorinating reaction eliminating the development of chlorinated organic compounds which are believed to increase the risk of cancer. These are called THMs (Trihalomethanes) or DBPs (Disinfection Bi-Products).
While chlorine dioxide (ClO2) includes the word chlorine, the two are very different. The difference between chlorine and ClO2 comes from the differences in their chemical structure.
Pi also manufacture and install chlorine analysers.
Chlorine is a very effective disinfectant for making and keeping drinking water safe, but when the drinking water source is surface water, containing organic materials. Chlorine dioxide is the best choice for a number of reasons:
- Chlorine dioxide is created on site, eliminating the need to store the chlorine and/or the transportation.
- Chlorine dioxide functions through an oxidative reaction rather than chlorinating reaction eliminating the development of chlorinated organic compounds which are believed to increase the risk of cancer. These are called THMs (Trihalomethanes) or DBPs (Disinfection Bi-Products).
Chlorine dioxide can be used as the main disinfectant for surface waters where there are issues with odour and taste. It acts as a successful biocide at concentrations as low as 0.1 ppm and over a wide pH range. Chlorine dioxide penetrates the cell wall of the bacteria and reacts with vital amino acids in the cytoplasm to kill the organism. The result of this reaction is chlorite. Chlorine dioxide is more widely used in industrial water treatment in applications such as food washing and hot water loops.
When handled correctly chlorine dioxide is a perfectly safe chemical to use. However, as with all disinfectant chemicals if eaten/drunk, subjected to prolonged exposure, handled improperly or absorbed through the skin the chemical can be toxic which in turn is what makes ClO2 such an effective water disinfectant.
Chlorine dioxide is environmentally friendly and can be used as a defence against pollution towards the environment or humans from bacteria and by-products from other disinfection methods.
Solutions of approximately 1% (10g/l) ClO2 can be stored at 5°C for several months, with little change in concentration. The solution must be stored in an airtight container with no exposure to light.
Chlorine dioxide can be made chemically from either sodium chlorite or sodium chlorate. It can also be generated electrochemically.
In comparison to chlorine, the cost of ClO2 is higher. The cost is dependent on the cost of the original chemicals (sodium chlorite or sodium chlorate), the chemicals used to change these chemicals into chlorine dioxide, and the generation technique used. The equipment for generating chlorine dioxide is also less expensive than that of other options that can be used for water treatment. In the instances where chlorine is not the preferred monitoring or environmental choice, chlorine dioxide is the most suitable solution.
There is no difference between a chlorine dioxide monitor and a chlorine dioxide analyser. They are just two expressions used interchangeably by different people. A chlorine dioxide controller is a chlorine dioxide monitor or analyser that has on board control functionality. The Pi DioSense monitors all have such on board functionality so for a Pi DioSense there is effectively no difference between a chlorine dioxide controller, analyser and monitor.
Focus Ons are a series of short articles distributed by email providing technical information regarding instrumentation, process measurement in potable, waste, process and pool waters. If you would like to join the mailing list, please contact us.
When discussing the disinfection of water, the use of chlorine (hypochlorite) will usually be one of the first topics discussed, however, did you know that…
…Pi provides the technology for the dosing, measurement, and control of an array of other disinfectants including hydrogen peroxide, peracetic acid and chlorine dioxide?
Chlorine dioxide, rather than simply being an alternative to chlorine, can be more suitable or effective for many industries and applications. This technology review will examine the properties and uses of chlorine dioxide and the methods that are used to measure its concentration in water.
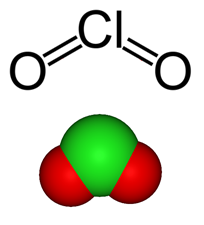
What is chlorine dioxide?
Chlorine dioxide is a compound with the formula ClO2. It appears as a yellowish-green gas and does not hydrolise in water, retaining its structure as a dissolved gas in solution. A powerful oxidiser, it was first suggested as a sterilising agent as early as 1900 but was only used on a plant scale as late as 19441.
The oxidising properties of chlorine dioxide make it useful for disinfection, and it has several advantages when compared to the more traditional chlorine. For example, it is more effective on specific pathogens such as Legionella2 or Giardia3.
When used properly, chlorine dioxide produces less harmful by-products than chlorine, and its disinfection ability is not influenced by pH. Its chemical properties mean that it can be cost effective because lower concentrations are needed compared to other disinfectants.
The uses of chlorine dioxide
 Although, historically, chlorine dioxide has been perceived as hazardous to handle (and in concentrated and gaseous forms, it certainly can be), modern on-site generation technology allows it to be used with more confidence and safety than ever before; as a result, chlorine dioxide has found its place in numerous applications:
Although, historically, chlorine dioxide has been perceived as hazardous to handle (and in concentrated and gaseous forms, it certainly can be), modern on-site generation technology allows it to be used with more confidence and safety than ever before; as a result, chlorine dioxide has found its place in numerous applications:
- Water disinfection (sewage, industrial processes, cooling towers, foodstuffs etc.)
- Bleaching wood, pulp and paper
- Biofilm removal due to its ability to penetrate the polysaccharide layers of bacterial capsules
- Oxidising soluble iron and manganese, allowing for easier removal
Just like all disinfection processes that take place at a plant scale, the need to balance proper disinfection and cost effectiveness means that accurate and reliable methods of measuring chlorine dioxide in water are needed.
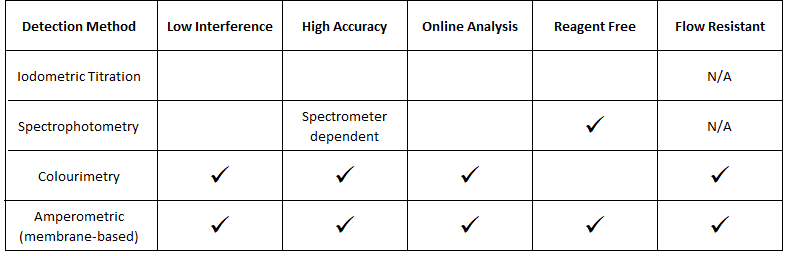
Several methods have been developed for this purpose, based upon a variety of different principles. These include:
- Iodometric Titration
- Spectrophotometry
- Colourimetry
- Membrane Amperometry
Iodometric Titration
 This method relies upon ClO2 oxidising iodine ions to iodine, which can then be titrated with sodium thiosulphate. Some relatively simple calculations can then be performed, and the concentration of chlorine dioxide can be deduced.
This method relies upon ClO2 oxidising iodine ions to iodine, which can then be titrated with sodium thiosulphate. Some relatively simple calculations can then be performed, and the concentration of chlorine dioxide can be deduced.
Advantages:
- Adjusting the pH allows the user to distinguish between Cl2 (chlorine), ClO2 (chlorine dioxide), ClO2⁻ (chlorite) and ClO3⁻ (chlorate). This makes the iodometric method ideal for measuring different chlorine species4
Disadvantages:
- Titrations can be difficult and time consuming to perform, and a higher level of technical skill is required than for other methods
- Chlorine dioxide, chlorite and hypochlorite are not easily distinguished which can cause problems in some applications
- Significant errors can arise if more than one species is present
- Although available as a test kit, the iodometric method has not been adapted for online analysis
Spectrophotometry
 This method is based upon the transmission of light. A specific wavelength of light (around 360nm in the case of chlorine dioxide) is passed through a cuvette containing the sample, and the transmission/absorption is measured.
This method is based upon the transmission of light. A specific wavelength of light (around 360nm in the case of chlorine dioxide) is passed through a cuvette containing the sample, and the transmission/absorption is measured.
The higher the concentration of chlorine dioxide, the lower the level of transmission; this forms the basis of the measurement.
Advantages:
- This method is relatively free of interferences compared to iodometric titration
- Spectrophotometers are easy to operate, and there is the potential for high accuracy
Disadvantages:
- The overall accuracy is dependent on the photometer used, with higher quality models costing thousands of pounds
- There is a known interference with chlorite, which can introduce significant inaccuracy5
- Online spectrophotometers exist but they aren’t designed with chlorine dioxide in mind
Colorimetric
 Colorimetric analysis is based on the reaction between ClO2 and a dye, and the measurement of the absorbance of a specific wavelength of light after this reaction takes place. The dyes that can be used include N.N-diethyl-p-phenylenediamine (DPD), chlorophenol red (CPR) and Lissamine Green (LGB). Each of these dyes has its own limitations (e.g. the color of the DPD changes over time, LGB is temperature dependent), but they also have their own positive characteristics.
Colorimetric analysis is based on the reaction between ClO2 and a dye, and the measurement of the absorbance of a specific wavelength of light after this reaction takes place. The dyes that can be used include N.N-diethyl-p-phenylenediamine (DPD), chlorophenol red (CPR) and Lissamine Green (LGB). Each of these dyes has its own limitations (e.g. the color of the DPD changes over time, LGB is temperature dependent), but they also have their own positive characteristics.
Advantages:
- Colorimetry has higher potential accuracy than spectrophotometry when performed well6. These kinds of tests are well established and are familiar to many technical and site staff
- Online colorimetry products are readily available from a variety of manufacturers
Disadvantages:
- The working range of the test is limited by the concentration of the dye, which places physiochemical constraints on what is possible7
- The purity of the dyes can be a problem, leading to inaccuracies; some studies report the purity of the same dye ranging from 45% to 95%
- Some dyes are not well suited to real time measurements due to longer reaction times
- Online colorimeters require a continual supply of reagents, which can be costly, and blockages in pipes can be quite common
Membrane based amperometric sensors (available from Pi)
 Designed specifically with online measurement in mind, these electrode-based sensors use a specialised electrolyte held within a membrane cap; this membrane allows ClO2 to diffuse into the electrolyte, where the following takes place at the electrodes:
Designed specifically with online measurement in mind, these electrode-based sensors use a specialised electrolyte held within a membrane cap; this membrane allows ClO2 to diffuse into the electrolyte, where the following takes place at the electrodes:
- Cathode: ClO2 + 4H⁺ + 5e⁻ → Cl⁻ + 2H2O
- Anode: Cl⁻ + Ag → AgCl + e⁺
The release of electrons at one electrode and the acceptance of electrons at the other creates a current flow between them, which forms the basis of the measurement.
Advantages:
- The membrane keeps harmful contaminants away from the electrodes, protecting them
- There is a low dependence on flow rate
- The electrolyte is pre-defined, and so the absence of chlorine dioxide gives zero current; this allows for a one-point calibration method
- No reagents or complex testing methods are required, meaning low operational costs over the lifetime of the sensor
- Continual operation makes these sensors ideal for online measurement
Disadvantages:
- Surfactants and detergents can affect the membrane, allowing water to pass into the electrolyte and cause signal drift
- Water repellent substances such as mineral oils can clog the membrane pores, so chlorine dioxide cannot pass into the electrolyte causing a measurement error
- There is a known ozone interference
How do Process Instruments approach the measurement of chlorine dioxide?
 All the approaches above have their own strengths and weaknesses and so the ‘ideal method’ depends upon the need of the end user.
All the approaches above have their own strengths and weaknesses and so the ‘ideal method’ depends upon the need of the end user.
As a water instrumentation and control company, Pi sees chlorine dioxide being used most in the kinds of applications that favour continual online analysis, and so the natural choice for Process Instruments is amperometric sensors.
Pi’s amperometric DioSense chlorine dioxide analysers allow for the responsive, real-time measurement of chlorine dioxide without the complex maintenance and reagents needed by online colorimetric systems. Their reagentless operation also means much lower total life costs; a large UK utility company who replaced 330 colorimetric sensors with amperometric probes saw savings of close to a million pounds over a ten year period.
Membrane based amperometric probes don’t require the strict flow control required by non-membrane systems, which can be difficult in some applications and industries. Provided some basic conditions are met (such as a lack of surfactants and detergents in the process stream), membrane based sensors are simple to install, calibrate and maintain.
References
- US Environmental Protection Agency: Office of Water, Alternative Disinfectants and Oxidants Manual, chapter 4: Chlorine Dioxide (EPA, 1999)
- Zhang, Z.; McCann, C.; Stout, J.; Piesczynski, S.; Hawks, R.; Vidic, R.; Yu, V. “Safety and Efficacy of Chlorine Dioxide for Legionella control in a Hospital Water System” (2007). Infection Control and Hospital Epidemiology. 28 (8): 1009-1012
- US Environmental Protection Agency: Office of Water, Alternative Disinfectants and Oxidants Manual, chapter 4: Chlorine Dioxide (EPA, 1999)
- Körtvélyesi, Z. Analytical Methods for The Measurement of Chlorine Dioxide and Related Oxychlorine Species in Aqueous Solution (University of Miami, 2004)
- Gordon, G. & Körtvélyesi, Z. “Chlorite Ion Interference in The Spectrophotometric Measurement of Chlorine Dioxide” (2004). Journal (American Water Works Association) 96 (9): 81-87
- Min Song et al. “Measurement of Chlorine Dioxide in Water by DPD Colorimeric Method” (2018) IOP Conf. Ser.: Earth Environ. Sci. 108 042102
- Körtvélyesi, Z. Analytical Methods for The Measurement of Chlorine Dioxide and Related Oxychlorine Species in Aqueous Solution (University of Miami, 2004)
Membraned sensors come with many advantages over non-membraned sensors such as higher resolutions, fewer interferents and a greatly reduced effect of flow rate changes. These advantages can make a huge difference to the bottom line, particularly if the cost of the chemical being dosed is quite high. For free chlorine sensors, using a membrane can make your measurement much less dependent on pH (if you are using sensors from Pi), meaning your measurement is a more accurate reflection of residual chlorine levels.
As such, membraned sensors are now largely the norm in residual chlorine measurement and are also prevalent for chlorine dioxide and ozone monitors, but did you know that…
…membraned sensors are sensitive to changes in pressure?
…flow cell outlets can airlock even when water is flowing through them?
…membraned sensors can still be used when the outlet does not go to drain?
…Pi has engineered and designed solutions to all of these potential issues?

Sensitivity to pressure
Membraned sensors do have one property that needs to be carefully managed; they are sensitive to pressure. Pi was an early adopter of membrane technology, so we know that the installation of these sensors is just as important as the sensor itself. In fact, the same sensors in different flow cells can give very different results.
In order to prevent pressure variations affecting the probe, Pi typically uses open flow cells which eliminate variability in pressure before it reaches the probe. Installers who are used to inline measurement and closed flow cells can sometimes run into problems managing the flow into and out of the cell.
Flow
Whether the sample to the cell is pumped, gravity fed, or comes from a pressurised line, it is important that the flow is controlled to within a range of 350-1000ml per minute, to ensure that sufficient flow is reaching the sensor and to prevent the flow cell overflowing.
If the flow to the cell is variable, Pi can provide a dole valve which controls the flow to approximately 500ml per minute, which prevents the cell from overflowing when pressure variations mean more flow than the cell can handle, while also ensuring adequate flow when the sample line flow/pressure reduces.
Airlocks
 The outlet of the flow cell needs to be open to atmosphere, and completely unobstructed. Any system with a long outlet line (particularly flexible pipe) is prone to get airlocks, which will cause the cell to overflow. Outlets which are visually clear and even have water flowing through them, can be partially airlocked which causes backpressure to overflow the cell. This is very easy to diagnose as if you see the cell overflowing and remove the outlet pipe, you will see the cell go back to normal operations within approximately 10 seconds. If this is a persistent problem, consider putting in an air break using a commercially available tundish.
The outlet of the flow cell needs to be open to atmosphere, and completely unobstructed. Any system with a long outlet line (particularly flexible pipe) is prone to get airlocks, which will cause the cell to overflow. Outlets which are visually clear and even have water flowing through them, can be partially airlocked which causes backpressure to overflow the cell. This is very easy to diagnose as if you see the cell overflowing and remove the outlet pipe, you will see the cell go back to normal operations within approximately 10 seconds. If this is a persistent problem, consider putting in an air break using a commercially available tundish.
Outlets that do not go to drain
The water from a membraned sensor doesn’t have to go to drain. For processes where saving water is a high priority, a simple tank and pump system that will pump sample water back into your main process line will allow water losses to be reduced to almost zero. Pi’s CRIUS®4.0 controller can be used to control this return process and ensure that this tank never overflows and can automatically drain itself periodically to avoid sediment build up.
What if things go wrong?
As any water engineer can tell you, no matter how well the system is designed, lines clog, pumps break and someone on site will fiddle with settings. Pi recognises these challenges and has engineered solutions into our systems. All Pi membraned sensor systems have the option to be able to:
- Have a flow switch to detect sample flow loss.
- Use dosing overfeed protection to protect against clogged dosing lines or pump failure.
- Have remote access for SMS or email alarms.
- Use relays to trigger beacons or sirens for alarms or control valves and pumps.
- Customise user security levels to control who can change what settings.
- Use status logs that show what happened to the system and when.
Conclusion
For membraned sensors, the best way of housing a membraned sensor is with an open flow cell. There are some occasions when this solution just isn’t practicable and in those instances the closed flow cells from Pi, which can take an overpressure of up to 3 bar, are the best solution. Please contact Pi for more details.
Many different sites ranging across the whole water use industry have a daily struggle to keep instrumentation functioning correctly due to sensor fouling. However, did you know that…
…self cleaning and self flushing systems are now available from Process Instruments for most types of sensors?
…these fouling removal systems can extend the life of sensors and drastically reduce maintenance?
…Pi’s self cleaning/flushing systems are affordable, simple and trouble free by design?
Sensor Fouling
 Whatever the process being monitored, there is often something in the sample water capable of fouling a sensor, and therefore causing erroneous results. The obvious solution to this problem is to clean the sensor, but how frequent should inspection and cleaning programs be for each piece of instrumentation? Too frequent and the inspection and cleaning regimen is time consuming and unnecessarily costly. Not often enough and the instrumentation will give false results and possibly fail prematurely.
Whatever the process being monitored, there is often something in the sample water capable of fouling a sensor, and therefore causing erroneous results. The obvious solution to this problem is to clean the sensor, but how frequent should inspection and cleaning programs be for each piece of instrumentation? Too frequent and the inspection and cleaning regimen is time consuming and unnecessarily costly. Not often enough and the instrumentation will give false results and possibly fail prematurely.
What is the solution?
Process Instruments’ AutoClean and AutoFlush Systems
Simple, reliable and easy to maintain, Process Instruments’ AutoClean/AutoFlush systems are an alternative to mechanical cleaning mechanisms which can clog and break. By frequently spraying the sensor/probe with clean water or air, the sensor remains clean and free from fouling for extended periods of time. The sensor cleaning cycle is activated by Pi’s controller for a user selectable length of time and frequency so that no matter how dirty the application, the probe remains clean. With no moving parts in the sensor body or in the cleaning attachment there is nothing to replace or check other than a simple valve positioned in an easy to reach location.
 Pi’s AutoClean and AutoFlush systems can give trouble free and fouling free functioning of sensors for weeks, if not months, at a time.
Pi’s AutoClean and AutoFlush systems can give trouble free and fouling free functioning of sensors for weeks, if not months, at a time.
A solution for each application
AutoClean
This option can be added to our pH, ORP, Turbidity, Suspended Solids and Dissolved Oxygen (DO) sensors. Consisting of an end cap to direct the flow of clean water (or air for a DO sensor) across the face of the sensor blasting any dirt away. The cleaning is controlled by a single valve positioned in an easily accessible location.
AutoVerify

If using air to clean a DO sensor the system can also automatically verify that the sensor is still responding correctly, removing any need to remove the sensor from the sample for months at a time.
AutoFlush
For sensors that require flow cell mounting like Chlorine, Ozone and Chlorine Dioxide,
an Autoflush system has inbuilt valves which automatically start/stop the sample flow and control the flow of clean water past the probe. The user can set the flushing interval and duration to keep the flow cell and sensor clear from fouling. For particularly dirty or stubborn contaminants, warm water can be used as the flush water to aid cleaning.
With the above options, whatever the application or parameter being measured, Process Instruments will be able to provide a monitoring system that will not only be accurate, precise and long lasting but that will also remain free from fouling and save the operator both time and money.
You probably know that most chlorine, ozone and chlorine dioxide analysers are calibrated using hand held DPD kits but did you know that…
…DPD can’t tell you when you have no residual?
…errors on DPD performance can be up to ± 100%?
…a significant number of service calls received by Pi relate to poor calibration?
What is DPD?
DPD (N.N-diethyl-p-phenylenediamine) is a chemical that when mixed with water containing an oxidant, changes colour depending on the concentration of the oxidant present. A handheld colorimeter measures light passing through the coloured solution. The absorption of that light by the liquid gives a concentration value. It is usually used to check concentration of, for example, free chlorine, total chlorine, ozone and chlorine dioxide etc. in water.
 When the DPD kit gives a value, it is often used to calibrate online instruments… and that is where Pi comes in!
When the DPD kit gives a value, it is often used to calibrate online instruments… and that is where Pi comes in!
As a manufacturer of online instruments we have to understand DPD in order to help our customers when they have problems calibrating their online monitors.
This Focus On will look at:
- The limitations of DPD (turbidity, zero oxidant, bleaching, pH and interferents).
- Minimising DPD measurement error (sampling, alignment and cleaning).
- Things to look out for (low concentrations, pink colour, stained glass).
- Little known chemistry (measuring bromine, chlorite versus chlorine dioxide).
- Rinse and repeat: is it really worth repeating my measurement?
What are the limitations of DPD?
DPD cannot measure below approximately 0.05 ppm.
 If you suspect there is zero oxidant in your sample, hold the vial up to a white surface. If you cannot see any trace of pink colour, it is likely any reading you are getting is from the unreacted DPD tablet.
If you suspect there is zero oxidant in your sample, hold the vial up to a white surface. If you cannot see any trace of pink colour, it is likely any reading you are getting is from the unreacted DPD tablet.
DPD cannot measure free chlorine above 6ppm
(and won’t always give a ‘high concentration’ reading error).
Many people are unaware that past a certain level of oxidant, DPD will not form its characteristic pink colour, and instead will ‘bleach’ to form a clear solution. This can lead people to think there is little or no oxidant in their water, when in fact there is so much that it is bleaching their DPD. Be on the lookout for a flash of pink when the tablet or powder is added if you suspect your sample is being bleached. NB. special kits and reagents are available for measuring oxidant above 6 ppm.
DPD cannot distinguish between oxidants such as:
chlorine, chlorine dioxide, chlorite, ozone, organochlorides, bromine and more, meaning interferents are a big problem.
DPD is a fantastic chemical, in that it is very versatile as a colouring agent, which is how it gives the oxidant the colour that we measure. This versatility does come at a price, DPD is not very specific as an analysis tool, and so if other chemicals are present in the sample, they can interfere with the reading, giving an inaccurate result. Common interferents include chlorine dioxide (for chlorine measurement, and vice versa), sodium chlorite, ozone, organochloramines, peroxides, and many more.
Minimising DPD measurement error
Here is an easy to read, printable checklist to ensure accurate DPD readings every time.
What To Watch Out for When Using DPD
Stained Glass
 The pink solution formed after DPD tests can leave a residue behind on the glass, which will affect the DPD reading. This residue can be easily cleaned off using what is in your DPD kit.
The pink solution formed after DPD tests can leave a residue behind on the glass, which will affect the DPD reading. This residue can be easily cleaned off using what is in your DPD kit.
Tap water
If you use normal tap water to wash out vials, droplets left behind can affect your reading due to the residual chlorine in drinking water. It is best (but not always practical) to use deionised water to wash out your vials, but if this isn’t available (deionised water can be purchased as car battery top up water from any car parts supplier) then you can use cooled boiled tap water, as boiling gets rid of any chlorine. If not then simply make sure the vials are perfectly dry before use.
Little Known Chemistry
DPD has a wide range of interferents. This means recurrent problems can sometimes be caused by the chemical makeup of the sample. For example, chlorite (ClO2–) and chlorine dioxide both affect DPD, but only chlorine dioxide is measured by most chlorine dioxide amperometric sensors. DPD can be used to track bromine, but DPD No.1 tablets measure FREE chlorine or TOTAL bromine. As combined bromine is just as effective a disinfectant as free bromine, this generally doesn’t pose too much of a problem, however some amperometric sensors measure free bromine, and cannot be calibrated using DPD No.1 tablets. For more information on measuring bromine, or chlorine in seawater, please see Pi’s technical note on Seawater Chlorination. Manganese and iron are both interferents with DPD and both are often present in drinking water treatment plants.
When discussing the disinfection of water, the use of chlorine (hypochlorite) will usually be one of the first topics discussed, however, did you know that…
…Pi provides the technology for the dosing, measurement, and control of an array of other disinfectants including hydrogen peroxide, peracetic acid and chlorine dioxide?
Chlorine dioxide, rather than simply being an alternative to chlorine, can be more suitable or effective for many industries and applications. This technology review will examine the properties and uses of chlorine dioxide and the methods that are used to measure its concentration in water.
What is chlorine dioxide?
Chlorine dioxide is a compound with the formula ClO2. It appears as a yellowish-green gas and does not hydrolise in water, retaining its structure as a dissolved gas in solution. A powerful oxidiser, it was first suggested as a sterilising agent as early as 1900 but was only used on a plant scale as late as 19441.
The oxidising properties of chlorine dioxide make it useful for disinfection, and it has several advantages when compared to the more traditional chlorine. For example, it is more effective on specific pathogens such as Legionella2 or Giardia3.
When used properly, chlorine dioxide produces less harmful by-products than chlorine, and its disinfection ability is not influenced by pH. Its chemical properties mean that it can be cost effective because lower concentrations are needed compared to other disinfectants.
The uses of chlorine dioxide
 Although, historically, chlorine dioxide has been perceived as hazardous to handle (and in concentrated and gaseous forms, it certainly can be), modern on-site generation technology allows it to be used with more confidence and safety than ever before; as a result, chlorine dioxide has found its place in numerous applications:
Although, historically, chlorine dioxide has been perceived as hazardous to handle (and in concentrated and gaseous forms, it certainly can be), modern on-site generation technology allows it to be used with more confidence and safety than ever before; as a result, chlorine dioxide has found its place in numerous applications:
- Water disinfection (sewage, industrial processes, cooling towers, foodstuffs etc.)
- Bleaching wood, pulp and paper
- Biofilm removal due to its ability to penetrate the polysaccharide layers of bacterial capsules
- Oxidising soluble iron and manganese, allowing for easier removal
Just like all disinfection processes that take place at a plant scale, the need to balance proper disinfection and cost effectiveness means that accurate and reliable methods of measuring chlorine dioxide in water are needed.
Several methods have been developed for this purpose, based upon a variety of different principles. These include:
- Iodometric Titration
- Spectrophotometry
- Colourimetry
- Membrane Amperometry
Iodometric Titration
 This method relies upon ClO2 oxidising iodine ions to iodine, which can then be titrated with sodium thiosulphate. Some relatively simple calculations can then be performed, and the concentration of chlorine dioxide can be deduced.
This method relies upon ClO2 oxidising iodine ions to iodine, which can then be titrated with sodium thiosulphate. Some relatively simple calculations can then be performed, and the concentration of chlorine dioxide can be deduced.
Advantages:
- Adjusting the pH allows the user to distinguish between Cl2 (chlorine), ClO2 (chlorine dioxide), ClO2⁻ (chlorite) and ClO3⁻ (chlorate). This makes the iodometric method ideal for measuring different chlorine species4
Disadvantages:
- Titrations can be difficult and time consuming to perform, and a higher level of technical skill is required than for other methods
- Chlorine dioxide, chlorite and hypochlorite are not easily distinguished which can cause problems in some applications
- Significant errors can arise if more than one species is present
- Although available as a test kit, the iodometric method has not been adapted for online analysis
Spectrophotometry
 This method is based upon the transmission of light. A specific wavelength of light (around 360nm in the case of chlorine dioxide) is passed through a cuvette containing the sample, and the transmission/absorption is measured.
This method is based upon the transmission of light. A specific wavelength of light (around 360nm in the case of chlorine dioxide) is passed through a cuvette containing the sample, and the transmission/absorption is measured.
The higher the concentration of chlorine dioxide, the lower the level of transmission; this forms the basis of the measurement.
Advantages:
- This method is relatively free of interferences compared to iodometric titration
- Spectrophotometers are easy to operate, and there is the potential for high accuracy
Disadvantages:
- The overall accuracy is dependent on the photometer used, with higher quality models costing thousands of pounds
- There is a known interference with chlorite, which can introduce significant inaccuracy5
- Online spectrophotometers exist but they aren’t designed with chlorine dioxide in mind
Colorimetric
 Colorimetric analysis is based on the reaction between ClO2 and a dye, and the measurement of the absorbance of a specific wavelength of light after this reaction takes place. The dyes that can be used include N.N-diethyl-p-phenylenediamine (DPD), chlorophenol red (CPR) and Lissamine Green (LGB). Each of these dyes has its own limitations (e.g. the color of the DPD changes over time, LGB is temperature dependent), but they also have their own positive characteristics.
Colorimetric analysis is based on the reaction between ClO2 and a dye, and the measurement of the absorbance of a specific wavelength of light after this reaction takes place. The dyes that can be used include N.N-diethyl-p-phenylenediamine (DPD), chlorophenol red (CPR) and Lissamine Green (LGB). Each of these dyes has its own limitations (e.g. the color of the DPD changes over time, LGB is temperature dependent), but they also have their own positive characteristics.
Advantages:
- Colorimetry has higher potential accuracy than spectrophotometry when performed well6. These kinds of tests are well established and are familiar to many technical and site staff
- Online colorimetry products are readily available from a variety of manufacturers
Disadvantages:
- The working range of the test is limited by the concentration of the dye, which places physiochemical constraints on what is possible7
- The purity of the dyes can be a problem, leading to inaccuracies; some studies report the purity of the same dye ranging from 45% to 95%
- Some dyes are not well suited to real time measurements due to longer reaction times
- Online colorimeters require a continual supply of reagents, which can be costly, and blockages in pipes can be quite common
Membrane based amperometric sensors (available from Pi)
 Designed specifically with online measurement in mind, these electrode-based sensors use a specialised electrolyte held within a membrane cap; this membrane allows ClO2 to diffuse into the electrolyte, where the following takes place at the electrodes:
Designed specifically with online measurement in mind, these electrode-based sensors use a specialised electrolyte held within a membrane cap; this membrane allows ClO2 to diffuse into the electrolyte, where the following takes place at the electrodes:
- Cathode: ClO2 + 4H⁺ + 5e⁻ → Cl⁻ + 2H2O
- Anode: Cl⁻ + Ag → AgCl + e⁺
The release of electrons at one electrode and the acceptance of electrons at the other creates a current flow between them, which forms the basis of the measurement.
Advantages:
- The membrane keeps harmful contaminants away from the electrodes, protecting them
- There is a low dependence on flow rate
- The electrolyte is pre-defined, and so the absence of chlorine dioxide gives zero current; this allows for a one-point calibration method
- No reagents or complex testing methods are required, meaning low operational costs over the lifetime of the sensor
- Continual operation makes these sensors ideal for online measurement
Disadvantages:
- Surfactants and detergents can affect the membrane, allowing water to pass into the electrolyte and cause signal drift
- Water repellent substances such as mineral oils can clog the membrane pores, so chlorine dioxide cannot pass into the electrolyte causing a measurement error
- There is a known ozone interference
How do Process Instruments approach the measurement of chlorine dioxide?
 All the approaches above have their own strengths and weaknesses and so the ‘ideal method’ depends upon the need of the end user.
All the approaches above have their own strengths and weaknesses and so the ‘ideal method’ depends upon the need of the end user.
As a water instrumentation and control company, Pi sees chlorine dioxide being used most in the kinds of applications that favour continual online analysis, and so the natural choice for Process Instruments is amperometric sensors.
Pi’s amperometric DioSense chlorine dioxide analysers allow for the responsive, real-time measurement of chlorine dioxide without the complex maintenance and reagents needed by online colorimetric systems. Their reagentless operation also means much lower total life costs; a large UK utility company who replaced 330 colorimetric sensors with amperometric probes saw savings of close to a million pounds over a ten year period.
Membrane based amperometric probes don’t require the strict flow control required by non-membrane systems, which can be difficult in some applications and industries. Provided some basic conditions are met (such as a lack of surfactants and detergents in the process stream), membrane based sensors are simple to install, calibrate and maintain.
References
- US Environmental Protection Agency: Office of Water, Alternative Disinfectants and Oxidants Manual, chapter 4: Chlorine Dioxide (EPA, 1999)
- Zhang, Z.; McCann, C.; Stout, J.; Piesczynski, S.; Hawks, R.; Vidic, R.; Yu, V. “Safety and Efficacy of Chlorine Dioxide for Legionella control in a Hospital Water System” (2007). Infection Control and Hospital Epidemiology. 28 (8): 1009-1012
- US Environmental Protection Agency: Office of Water, Alternative Disinfectants and Oxidants Manual, chapter 4: Chlorine Dioxide (EPA, 1999)
- Körtvélyesi, Z. Analytical Methods for The Measurement of Chlorine Dioxide and Related Oxychlorine Species in Aqueous Solution (University of Miami, 2004)
- Gordon, G. & Körtvélyesi, Z. “Chlorite Ion Interference in The Spectrophotometric Measurement of Chlorine Dioxide” (2004). Journal (American Water Works Association) 96 (9): 81-87
- Min Song et al. “Measurement of Chlorine Dioxide in Water by DPD Colorimeric Method” (2018) IOP Conf. Ser.: Earth Environ. Sci. 108 042102
- Körtvélyesi, Z. Analytical Methods for The Measurement of Chlorine Dioxide and Related Oxychlorine Species in Aqueous Solution (University of Miami, 2004)
Membraned sensors come with many advantages over non-membraned sensors such as higher resolutions, fewer interferents and a greatly reduced effect of flow rate changes. These advantages can make a huge difference to the bottom line, particularly if the cost of the chemical being dosed is quite high. For free chlorine sensors, using a membrane can make your measurement much less dependent on pH (if you are using sensors from Pi), meaning your measurement is a more accurate reflection of chlorine residual.
As such, membraned sensors are now largely the norm in residual chlorine measurement and are also prevalent for chlorine dioxide and ozone monitors, but did you know that…
…membraned sensors are sensitive to changes in pressure?
…flow cell outlets can airlock even when water is flowing through them?
…membraned sensors can still be used when the outlet does not go to drain?
…Pi has engineered and designed solutions to all of these potential issues?
Sensitivity to pressure
Membraned sensors do have one property that needs to be carefully managed; they are sensitive to pressure. Pi was an early adopter of membrane technology, so we know that the installation of these sensors is just as important as the sensor itself. In fact, the same sensors in different flow cells can give very different results.
In order to prevent pressure variations affecting the probe, Pi typically uses open flow cells which eliminate variability in pressure before it reaches the probe. Installers who are used to inline measurement and closed flow cells can sometimes run into problems managing the flow into and out of the cell.
Flow
Whether the sample to the cell is pumped, gravity fed, or comes from a pressurised line, it is important that the flow is controlled to within a range of 350-1000ml per minute, to ensure that sufficient flow is reaching the sensor and to prevent the flow cell overflowing.
If the flow to the cell is variable, Pi can provide a dole valve which controls the flow to approximately 500ml per minute, which prevents the cell from overflowing when pressure variations mean more flow than the cell can handle, while also ensuring adequate flow when the sample line flow/pressure reduces.
Airlocks
The outlet of the flow cell needs to be open to atmosphere, and completely unobstructed. Any system with a long outlet line (particularly flexible pipe) is prone to get airlocks, which will cause the cell to overflow. Outlets which are visually clear and even have water flowing through them, can be partially airlocked which causes backpressure to overflow the cell. This is very easy to diagnose as if you see the cell overflowing and remove the outlet pipe, you will see the cell go back to normal operations within approximately 10 seconds. If this is a persistent problem, consider putting in an air break using a commercially available tundish.
Outlets that do not go to drain
The water from a membraned sensor doesn’t have to go to drain. For processes where saving water is a high priority, a simple tank and pump system that will pump sample water back into your main process line will allow water losses to be reduced to almost zero. Pi’s CRIUS®4.0 controller can be used to control this return process and ensure that this tank never overflows and can automatically drain itself periodically to avoid sediment build up.
What if things go wrong?
As any water engineer can tell you, no matter how well the system is designed, lines clog, pumps break and someone on site will fiddle with settings. Pi recognises these challenges and has engineered solutions into our systems. All Pi membraned sensor systems have the option to be able to:
- Have a flow switch to detect sample flow loss.
- Use dosing overfeed protection to protect against clogged dosing lines or pump failure.
- Have remote access for SMS or email alarms.
- Use relays to trigger beacons or sirens for alarms or control valves and pumps.
- Customise user security levels to control who can change what settings.
- Use status logs that show what happened to the system and when.
Conclusion
For membraned sensors, the best way of housing a membraned sensor is with an open flow cell. There are some occasions when this solution just isn’t practicable and in those instances the closed flow cells from Pi, which can take an overpressure of up to 3 bar, are the best solution. Please contact Pi for more details.
Measuring free chlorine and chlorine dioxide independently of each other is quite a challenge, given their chemical similarities. Many sensors struggle to differentiate between the two measurands, but did you know that…
…many chlorine probes suffer from interference in the presence of chlorine dioxide?
…DPD1 will read both chlorine and chlorine dioxide?
…you can have accurate chlorine dioxide control in water where chlorine is present?
Free chlorine and chlorine dioxide are both oxidants used for disinfection in water. Each act differently as a disinfectant but are measured in almost the same way; with an electrochemical sensor or with an online DPD sensor. It is sometimes beneficial to have both disinfectants in the water at the same time, particularly when chlorine dioxide is being added to mains water.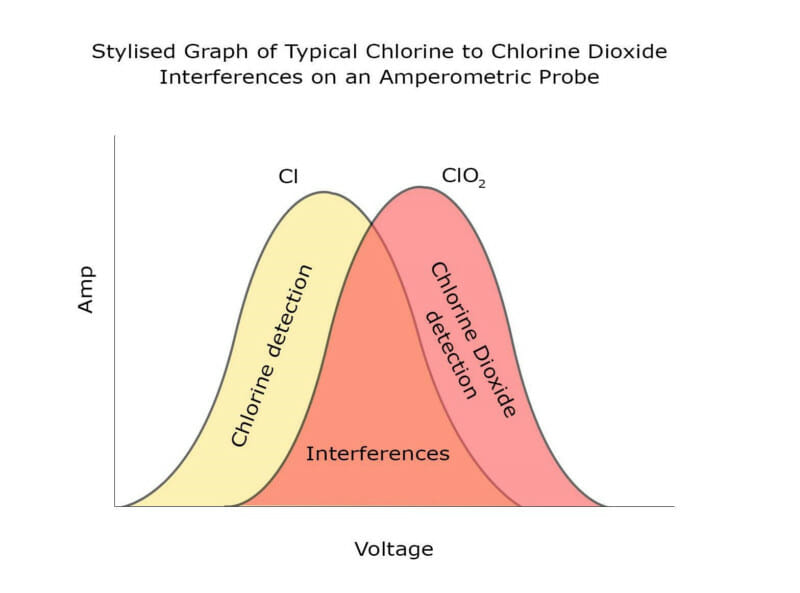 In most non-membraned (and some membraned) amperometric sensors, oxidants are detected by a current produced at the working electrode, at a particular voltage. The same technology can effectively be ‘tuned’ to different oxidisers by varying the voltage. Lots of oxidants are measurable over a range of voltages, and sometimes those response curves overlap.
In most non-membraned (and some membraned) amperometric sensors, oxidants are detected by a current produced at the working electrode, at a particular voltage. The same technology can effectively be ‘tuned’ to different oxidisers by varying the voltage. Lots of oxidants are measurable over a range of voltages, and sometimes those response curves overlap.
The graph shows that at almost any voltage where you can measure free chlorine, the chlorine dioxide curve overlaps with the chlorine one. This means it can be very difficult to find a probe that measures free chlorine, but doesn’t measure chlorine dioxide. Although these response curves can shift depending on probe design, electrode material and other factors, it is very difficult to engineer a response curve that gives a good signal for free chlorine but not for chlorine dioxide. The DioSense membraned chlorine dioxide sensor from Pi is not susceptible to interference by free chlorine. This means that the DioSense sensor can be used in conjunction with Pi’s HaloSense free chlorine sensor, to measure chlorine dioxide and free chlorine independently of each other in the same application, on Pi’s CRONOS® or CRIUS®4.0 analyser. The analyser takes the signal from the free chlorine probe, which does have a known interference from chlorine dioxide (1ppm of chlorine dioxide will show up as 0.75ppm of chlorine). The normalised signal from the chlorine dioxide sensor can then be removed.
The DioSense membraned chlorine dioxide sensor from Pi is not susceptible to interference by free chlorine. This means that the DioSense sensor can be used in conjunction with Pi’s HaloSense free chlorine sensor, to measure chlorine dioxide and free chlorine independently of each other in the same application, on Pi’s CRONOS® or CRIUS®4.0 analyser. The analyser takes the signal from the free chlorine probe, which does have a known interference from chlorine dioxide (1ppm of chlorine dioxide will show up as 0.75ppm of chlorine). The normalised signal from the chlorine dioxide sensor can then be removed.
Sensor Fouling
 Whatever the process being monitored, there is often something in the sample water capable of fouling a sensor, and therefore causing erroneous results. The obvious solution to this problem is to clean the sensor, but how frequent should inspection and cleaning programs be for each piece of instrumentation? Too frequent and the inspection and cleaning regimen is time consuming and unnecessarily costly. Not often enough and the instrumentation will give false results and possibly fail prematurely.
Whatever the process being monitored, there is often something in the sample water capable of fouling a sensor, and therefore causing erroneous results. The obvious solution to this problem is to clean the sensor, but how frequent should inspection and cleaning programs be for each piece of instrumentation? Too frequent and the inspection and cleaning regimen is time consuming and unnecessarily costly. Not often enough and the instrumentation will give false results and possibly fail prematurely.
What is the solution?
Process Instruments’ AutoClean and AutoFlush Systems
Simple, reliable and easy to maintain, Process Instruments’ AutoClean/AutoFlush systems are an alternative to mechanical cleaning mechanisms which can clog and break. By frequently spraying the sensor/probe with clean water or air, the sensor remains clean and free from fouling for extended periods of time. The sensor cleaning cycle is activated by Pi’s controller for a user selectable length of time and frequency so that no matter how dirty the application, the probe remains clean. With no moving parts in the sensor body or in the cleaning attachment there is nothing to replace or check other than a simple valve positioned in an easy to reach location. Pi’s AutoClean and AutoFlush systems can give trouble free and fouling free functioning of sensors for weeks, if not months, at a time.
Pi’s AutoClean and AutoFlush systems can give trouble free and fouling free functioning of sensors for weeks, if not months, at a time.
A solution for each application
AutoClean
This option can be added to our pH, ORP, Turbidity, Suspended Solids and Dissolved Oxygen (DO) sensors. Consisting of an end cap to direct the flow of clean water (or air for a DO sensor) across the face of the sensor blasting any dirt away. The cleaning is controlled by a single valve positioned in an easily accessible location.AutoVerify
 If using air to clean a DO sensor the system can also automatically verify that the sensor is still responding correctly, removing any need to remove the sensor from the sample for months at a time.
If using air to clean a DO sensor the system can also automatically verify that the sensor is still responding correctly, removing any need to remove the sensor from the sample for months at a time.
AutoFlush
For sensors that require flow cell mounting like Chlorine, Ozone and Chlorine Dioxide, an Autoflush system has inbuilt valves which automatically start/stop the sample flow and control the flow of clean water past the probe. The user can set the flushing interval and duration to keep the flow cell and sensor clear from fouling. For particularly dirty or stubborn contaminants, warm water can be used as the flush water to aid cleaning. With the above options, whatever the application or parameter being measured, Process Instruments will be able to provide a monitoring system that will not only be accurate, precise and long lasting but that will also remain free from fouling and save the operator both time and money.You probably know that most chlorine, ozone and chlorine dioxide analysers are calibrated using hand held DPD kits but did you know that…
…DPD can’t tell you when you have no residual?
…errors on DPD performance can be up to ± 100%?
…a significant number of service calls received by Pi relate to poor calibration?
What is DPD?
DPD (N.N-diethyl-p-phenylenediamine) is a chemical that when mixed with water containing an oxidant, changes colour depending on the concentration of the oxidant present. A handheld colorimeter measures light passing through the coloured solution. The absorption of that light by the liquid gives a concentration value. It is usually used to check concentration of, for example, free chlorine, total chlorine, ozone and chlorine dioxide etc. in water.
 When the DPD kit gives a value, it is often used to calibrate online instruments… and that is where Pi comes in!
When the DPD kit gives a value, it is often used to calibrate online instruments… and that is where Pi comes in!
As a manufacturer of online instruments we have to understand DPD in order to help our customers when they have problems calibrating their online monitors.
This Focus On will look at:
- The limitations of DPD (turbidity, zero oxidant, bleaching, pH and interferents).
- Minimising DPD measurement error (sampling, alignment and cleaning).
- Things to look out for (low concentrations, pink colour, stained glass).
- Little known chemistry (measuring bromine, chlorite versus chlorine dioxide).
- Rinse and repeat: is it really worth repeating my measurement?
What are the limitations of DPD?
DPD cannot measure below approximately 0.05 ppm.
 If you suspect there is zero oxidant in your sample, hold the vial up to a white surface. If you cannot see any trace of pink colour, it is likely any reading you are getting is from the unreacted DPD tablet.
If you suspect there is zero oxidant in your sample, hold the vial up to a white surface. If you cannot see any trace of pink colour, it is likely any reading you are getting is from the unreacted DPD tablet.
DPD cannot measure free chlorine above 6ppm
(and won’t always give a ‘high concentration’ reading error).
Many people are unaware that past a certain level of oxidant, DPD will not form its characteristic pink colour, and instead will ‘bleach’ to form a clear solution. This can lead people to think there is little or no oxidant in their water, when in fact there is so much that it is bleaching their DPD. Be on the lookout for a flash of pink when the tablet or powder is added if you suspect your sample is being bleached. NB. special kits and reagents are available for measuring oxidant above 6 ppm.
DPD cannot distinguish between oxidants such as:
chlorine, chlorine dioxide, chlorite, ozone, organochlorides, bromine and more, meaning interferents are a big problem.
DPD is a fantastic chemical, in that it is very versatile as a colouring agent, which is how it gives the oxidant the colour that we measure. This versatility does come at a price, DPD is not very specific as an analysis tool, and so if other chemicals are present in the sample, they can interfere with the reading, giving an inaccurate result. Common interferents include chlorine dioxide (for chlorine measurement, and vice versa), sodium chlorite, ozone, organochloramines, peroxides, and many more.
Minimising DPD measurement error
Here is an easy to read, printable checklist to ensure accurate DPD readings every time.
What To Watch Out for When Using DPD
Stained Glass
 The pink solution formed after DPD tests can leave a residue behind on the glass, which will affect the DPD reading. This residue can be easily cleaned off using what is in your DPD kit.
The pink solution formed after DPD tests can leave a residue behind on the glass, which will affect the DPD reading. This residue can be easily cleaned off using what is in your DPD kit.
Tap water
If you use normal tap water to wash out vials, droplets left behind can affect your reading due to the residual chlorine in drinking water. It is best (but not always practical) to use deionised water to wash out your vials, but if this isn’t available (deionised water can be purchased as car battery top up water from any car parts supplier) then you can use cooled boiled tap water, as boiling gets rid of any chlorine. If not then simply make sure the vials are perfectly dry before use.
Little Known Chemistry
DPD has a wide range of interferents. This means recurrent problems can sometimes be caused by the chemical makeup of the sample. For example, chlorite (ClO2–) and chlorine dioxide both affect DPD, but only chlorine dioxide is measured by most chlorine dioxide amperometric sensors. DPD can be used to track bromine, but DPD No.1 tablets measure FREE chlorine or TOTAL bromine. As combined bromine is just as effective a disinfectant as free bromine, this generally doesn’t pose too much of a problem, however some amperometric sensors measure free bromine, and cannot be calibrated using DPD No.1 tablets. For more information on measuring bromine, or chlorine in seawater, please see Pi’s technical note on Seawater Chlorination. Manganese and iron are both interferents with DPD and both are often present in drinking water treatment plants.
| Document | Type | Size |
|---|---|---|
| DioSense | Brochure | 675kB |
| Chlorine Dioxide Measurements – A Technology Review | Article | 625kB |
| Using Open Flow Cells with Membraned Sensors | Technical Note | 762kB |
| DPD Checklist | Technical Note | 487kB |
| Probe Fouling | Technical Note | 459kB |
"We at Scottish Water have been using the excellent Pi LabSense 3 and portable UV254 instruments in the field for optimizing our Water Treatment processes for some years now. We find them easy to use and invaluable for detecting and resolving issues in a timely, efficient and effective manner. Two great pieces of kit!”
Paul Weir
Scottish Water - UK
"I want to thank you and all at Process Instruments for all the assistance, information and handholding during the year. The help that was so freely given was very much appreciated. Can you please extend my appreciation to all at Pi and let me wish you all a happy, safe and wonderful Christmas and I look forward to working with you again in 2021."
Michael Bailey
Wexford Co. Co. - Ireland
"Excellent level of support and always so much more prompt than a lot of our suppliers."
Phill Tuxford
Detectronic - UK
"The plant can’t produce good quality water without the CoagSense."
Mick Murphy
Wexford Co. Co. - Ireland
"We started using Process Instruments 10 years ago and they worked so well that when we were looking at expanding, Process Instruments were our "go to" company."
Rebecca - Pool Manager
Woodland Spa - Burnley, UK
"We've been installing pools and spa controllers from Pi for more than 10 years and they just are the best on the market."
Dr. Lester Symmonds
Pool Sentry - UK
"We've used the CRIUS® with chlorine, pH and conductivity sensors for several years and confirm quality, performance and reliability has been wholly satisfactory to date."
David Kerr
Karis Technical Services Ltd. - UK
"We in ECM ECO Monitoring can only recommend Process Instruments products and services to all other potential clients. They have very complex portfolio of products for water quality monitoring in various types of industries, friendly attitude, very quick delivery time and prompt reaction to all our needs and inquires. Our clients especially appreciate the particle counters and sizers allowing identification of drinking water treatment problems. The Streaming Current Monitors are a great tool for optimisation of expensive chemicals.”
Branislav
ECM ECO Monitoring - Slovakia
"Simply the best turbidity available."
John Clark
Chemtrac - Atlanta, GA
"In 2019, we purchased 29 particle counters which were installed in our water plants. So far, their performance has been perfect."
Li Yongjun
Jinan Hongquan Water Company - China
"We have installed hundreds of ozone analyzers from Pi over the years. They are just accurate, reliable and require low maintenance."
Jiao Tumei
Qingdao Guolin Environmental Technology Co., Ltd., - China
"Over the last few years we've purchased chlorine and turbidity analyzers from Chemtrac and with routine calibration the probes measure the chlorine and turbidity without any issue. We are very happy with this product and would highly recommend them."
Daniel "Buck" Owen
Ocoee Utility District - Ocoee TN
"We've been using these analyzers since 2008. They're easy to use and very stable. Calibration and maintenance is quick and simple."
Lloyd Gruginski
Chehalis WTP - Chehalis, WA
"The Pi products provide excellent value for money and represent the best municipal drinking water analyzers available."
John Clark
Chemtrac - Atlanta
“Servicing customers is much more than just solving problems or addressing complaints and Pi does that very competently with technical and quick efforts providing a good experience."
Clovis Tuchapski
Buckman - Latin America
"Pi's technical team has enabled us to be one step ahead of our competitors by adding value to our projects thanks to their fast and excellent support from the moment you first reach out.
Ibrahim Kaplin
Thermomed - Turkey
"Going from ORP control to amperometric chlorine sensor control has undoubtedly improved the pool water immensely!"
Chris Tedeschi
Link Automation - USA
"The Streaming Current Monitor from Pi is the best SCM I have ever used. The analyser responds quickly and has many powerful functions, which helps me save a lot of money."
Ye Yancong
Xiamen Xishan Water Plant - China
“Process Instruments has a broad range of high quality and user-friendly solutions for water-industry problems. The short lead times and great customer support make Pi a reliable partner.”
Péter Szabó
SC KATALIN NOHSE CHIMIST IMPORT SRL - Romania
"Process Instruments UK always have a high level of customer service. All our interactions with Pi have exceeded our expectations. It is always a pleasure working with you.”
Iñaki Seisdedos Rodríguez
Izasa Scientific - Spain
"The support from Pi and its partners is superb. They go above and beyond to ensure that, not only is their equipment perfect but that the process is working great too. Five Stars!"
Anthony Glitto
Equip Solutions - Illinois, USA
"Process Instruments UK always have a high level of customer service. All our interactions with Pi have exceeded our expectations. It is always a pleasure working with you.”
Rudi Tuffek
Allpronix - South Africa












