Chlorine Analysers For Residual Chlorine Monitoring – HaloSense
Free chlorine analysers and Total chlorine analysers measure the chlorine in water that is ‘residual’ (left over) after disinfection has happened. i.e. if you add chlorine to water it disinfects, and if there is any leftover, it is ‘residual’ and therefore available to disinfect more. This residual chlorine can be measured and used to give confidence that disinfection is complete. Pi has three free chlorine analysers and total chlorine analysers for measuring residual chlorine; HaloSense (that uses electrochemical sensors), DPDSense (that uses online DPD technology), and Chloribrid® (that uses a hybrid of both). Free chlorine refers to HOCl and OCl–, and total chlorine refers to free chlorine plus chloramines. Both free and total chlorine are effective disinfectants.

Chlorine Analyser
The HaloSense free chlorine analyser and HaloSense total chlorine analyser use three electrode amperometric sensors. Two of the electrodes (the gold working electrode and the silver/halide reference electrode) are behind a membrane that separates the sample from the electrodes and are submerged in an electrolyte. In the free chlorine analyser the membrane allows the movement of chlorine from the sample to inside the sensor where a low pH environment converts the vast majority of any OCl– present to HOCl. The HOCl is measured at the working electrode and a current proportional to the concentration of chlorine is produced which is reported back to the analyser. In the total chlorine analyser the free chlorine and combined chlorine migrate through the membrane where the chlorine is displaced by iodine, which is measured at the working electrode.
- Low purchase cost
- Low cost of ownership
- Reduced pH dependency (largely pH independent)
- Stable and reliable
- Bufferless
- Reagentless
Many water companies want to use a free chlorine analyser without the need for chemical buffers traditionally associated with such measurements. Acetate and phosphate buffers are expensive and environmentally unfriendly. Buffer delivery systems are maintenance intensive and have costly consumables, and there are health and safety considerations in the handling of the acids and high disposal costs if the acid treated water is unable to be fed back into the water supply.
Free Chlorine Analysers
Amperometric sensors and most polarographic probes only respond to hypochlorous acid, (HOCl). HOCl dissociates into hypochlorite (OCl–)pH dependently. This is why most chlorine monitors need acid buffers in most applications. The typical pH of water measured on a water treatment works may range from 7 to 9.2. Chemical buffering reduces the pH to between 5 and 6 and ensures that the majority of the free chlorine is present as HOCl (see graph below).
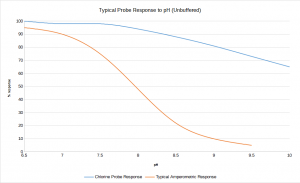 The HaloSense Free Chlorine Analyser measures all the HOCl and the majority of the OCl– present (blue line on graph). This results in a vastly reduced pH effect and means that most chlorine monitoring applications require no buffer and no pH compensation.
The HaloSense Free Chlorine Analyser measures all the HOCl and the majority of the OCl– present (blue line on graph). This results in a vastly reduced pH effect and means that most chlorine monitoring applications require no buffer and no pH compensation.
- Continuous online monitoring for free chlorine in any water
- Water treatment plant total chlorine analyser dosing control
- Secondary chlorination free chlorine dosing control
- Distribution monitoring
- Cooling tower monitoring and control
- Pasteuriser dosing control
- Seawater chlorination control
- Bromine monitoring in seawater
- Food washing
- Chloramination control
The HaloSense chlorine monitor range is particularly suited to working in sites where reliability and ease of use are most important.
 Small water treatment plants, secondary disinfection plants etc. tend to suffer from similar problems wherever they are in the world. The first is lack of communications SCADA infrastructure, the cost of which to install can be prohibitive. The second is the lack of a central DCS control infrastructure, again the installation of which can be cost prohibitive. The third is the remote location. Often these water treatment plants are in remote and difficult to access locations.
Small water treatment plants, secondary disinfection plants etc. tend to suffer from similar problems wherever they are in the world. The first is lack of communications SCADA infrastructure, the cost of which to install can be prohibitive. The second is the lack of a central DCS control infrastructure, again the installation of which can be cost prohibitive. The third is the remote location. Often these water treatment plants are in remote and difficult to access locations.
With these three issues facing many water engineers around the world, a low cost solution providing solutions to all three issues is available from Pi. A CRIUS®4.0 controller has the on board capacity to provide small scale SCADA, and full online PID control whilst the sensors (e.g. chlorine, pH, turbidity etc.) are suitable for long term operation without operator intervention.
To learn more about the control capability of the CRIUS®4.0 please click here.
To hear more about other customers using the CRIUS®4.0 multi-parameter controllers in a similar way why not contact us?
Each Residual Chlorine Analyser from Pi has the capability to be an extremely capable Chlorine Controller. The controllers can have multiple control channels which can utilise chemical control (usually a relay (switch) turns dosing on when the chlorine is too low or off when it is too high) or PID control.
PID stands for Proportional Integrated Derivative and it is a mathematical manipulation of the sensor signal to give an output that will control a pump and manage a constant chlorine level in the water. All the features are adjustable and there are safety features built in such as overfeed protection. For a discussion of PID control please see our technical notes here.
Pi’s chlorine controllers have been used in many control applications such as in pasteurisers, water treatment, cooling towers, swimming pools etc
The following are available in the HaloSense range;
- Free chlorine anlayser – 0.005-2ppm, 0.05-5ppm, 0.05-10ppm, 0.05-20ppm, 0.5-200ppm
- Total chlorine analyser – 0.005-0.5ppm, 0.005-2ppm, 0.05-5ppm, 0.05-10ppm, 0.05-20ppm
- Online chlorine analyser in seawater analysers (free or total bromine) – 0.005-2ppm, 0.05-5ppm, 0.05-10ppm, 0.05-20ppm
- Online zero chlorine analyser (designed to measure the absence of free chlorine) – 0.005-2ppm for applications such as post activated carbon and pre-RO monitoring.
Other options include;
The HaloSense sensors can come equipped to automatically clean themselves at user defined intervals, with all the benefits of no operator intervention for up to 6 months. The AutoFlush is particularly useful in food preparation, pulp and paper, and many applications where there is likely to be a build up of solids in the sample. For more information about AutoFlush click here.
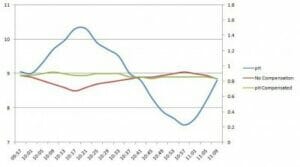 For some free chlorine applications with high and variable pH, pH compensation can improve the accuracy of the analyser. For pH compensation to be valid it must be done with the highest quality pH sensors and with chlorine sensors that have a reduced susceptibility to varying pH, such as those used in the HaloSense range.
For some free chlorine applications with high and variable pH, pH compensation can improve the accuracy of the analyser. For pH compensation to be valid it must be done with the highest quality pH sensors and with chlorine sensors that have a reduced susceptibility to varying pH, such as those used in the HaloSense range.
The graph shows the errors on a real HaloSense free chlorine sensor when a sample of 1 ppm free chlorine has the pH changed from pH 9 to more than pH 10, down to pH 7.5 and back again. The graph shows that the vast majority of applications won’t need pH compensation at all and for those that do that free chlorine sensor is the most appropriate sensor available to have that compensation applied.
The CRONOS® and CRIUS®4.0 free and total residual dosing controllers can be equipped with four PID process control options, data-logging, relay outputs, analog outputs and serial communications such as: Ethernet, Modbus and Profibus.
Remote monitoring of the instruments (including remote access to all control options) is available via the internet over GPRS and via a LAN. In fact the CRIUS®4.0 HaloSense monitor has all the options you could want, whilst the CRONOS® provides a low cost alternative and is particularly great value for money!
Pi offers Free and Total Chlorine sensors in the range 0.005-0.5ppm (total only), 0.005-2ppm, 0.05-5ppm, 0.05-10ppm, 0.05-20ppm and 0.5-200ppm (free only).
This depends on the application. The online chlorine sensor has a very low drift so most people calibrate it either once a week, once a month or even every six months.
Once a year (free and total), every 3-6 months (zero).
Once a year.
Yes, but only a very small amount and most users are happy to accept this.
Pi also design and install a range of pH analysers.
Both ozone and chlorine dioxide will interfere with the measurement. For more information, click here.
If stored in a cool dry place, two years.
PVC-U, stainless steel, hydrophilic membrane, PEEK (total and zero) and silicone.
0°C – 45°C (free and total), 0°C – 40°C (zero).
The sensor operates at a positive voltage all of the time so any drift on the zero is negligible compared to the positive operating voltage so no zero is necessary.
Nothing! The sensor has a thermistor that measures the temperature and does an automatic compensation.
Use a handheld meter. These are available from a variety of suppliers and nearly all of them utilise colourimetric DPD to determine the chlorine concentration in the sample.
Firstly take the sample from right at the instrument. Secondly don’t take the sample when the concentration is varying quickly, and thirdly use a good quality handheld and follow the instructions carefully.
During calibration the analyser looks at the stability (rate of change) of the signal from the probe and if it varies by more than 10% over the countdown then the analyser prevents calibration to avoid the calibration routine introducing errors.
Free chlorine reacts with things in a pool and changes them. For example free chlorine reacts with viruses and bacteria. It changes and kills those organisms but is changed by them in the same time…in effect it is used up. Free chlorine can also react with ammonia in a pool to form combined chlorine. No free chlorine in a pool is always a result of either there isn’t chlorine going into the pool (a dosing problem) or it has all been used up (reacted).
| Document | Type | Size |
|---|---|---|
| HaloSense | Brochure | 724kB |
| HaloSense Zero | Technical Note | 606kB |
| HaloSense Hints and Tips | Technical Note | 606kB |
| Potential Savings when Choosing Amperometric Chlorine Measurements over online DPD | Article | 525kB |
| Seawater Chlorination | Technical Note | 710kB |
| ORP vs. ppm | Technical Note | 534kB |
| pH Effects on Pi’s Free Chlorine Sensor | Technical Note | 702kB |
| pH Compensation of a Residual Chlorine Measurement | Technical Note | 540kB |
| Free Chlorine Probe Maintenance | Technical Note | 658kB |
| Total Chlorine Probe Maintenance | Technical Note | 689kB |
| Using Open and Closed Flow Cells with Membraned Sensors | Technical Note | 762kB |
| DPD Checklist | Technical Note | 487kB |
| Probe Fouling | Technical Note | 459kB |
"We at Scottish Water have been using the excellent Pi LabSense 3 and portable UV254 instruments in the field for optimizing our Water Treatment processes for some years now. We find them easy to use and invaluable for detecting and resolving issues in a timely, efficient and effective manner. Two great pieces of kit!”
Paul Weir
Scottish Water - UK
"I want to thank you and all at Process Instruments for all the assistance, information and handholding during the year. The help that was so freely given was very much appreciated. Can you please extend my appreciation to all at Pi and let me wish you all a happy, safe and wonderful Christmas and I look forward to working with you again in 2021."
Michael Bailey
Wexford Co. Co. - Ireland
"Excellent level of support and always so much more prompt than a lot of our suppliers."
Phill Tuxford
Detectronic - UK
"The plant can’t produce good quality water without the CoagSense."
Mick Murphy
Wexford Co. Co. - Ireland
"We've used the CRIUS® with chlorine, pH and conductivity sensors for several years and confirm quality, performance and reliability has been wholly satisfactory to date."
David Kerr
Karis Technical Services Ltd. - UK
"We in ECM ECO Monitoring can only recommend Process Instruments products and services to all other potential clients. They have very complex portfolio of products for water quality monitoring in various types of industries, friendly attitude, very quick delivery time and prompt reaction to all our needs and inquires. Our clients especially appreciate the particle counters and sizers allowing identification of drinking water treatment problems. The Streaming Current Monitors are a great tool for optimisation of expensive chemicals.”
Branislav
ECM ECO Monitoring - Slovakia
"Simply the best turbidity available."
John Clark
Chemtrac - Atlanta, GA
"In 2019, we purchased 29 particle counters which were installed in our water plants. So far, their performance has been perfect."
Li Yongjun
Jinan Hongquan Water Company - China
"We have installed hundreds of ozone analyzers from Pi over the years. They are just accurate, reliable and require low maintenance."
Jiao Tumei
Qingdao Guolin Environmental Technology Co., Ltd., - China
"Over the last few years we've purchased chlorine and turbidity analyzers from Chemtrac and with routine calibration the probes measure the chlorine and turbidity without any issue. We are very happy with this product and would highly recommend them."
Daniel "Buck" Owen
Ocoee Utility District - Ocoee TN
"We've been using these analyzers since 2008. They're easy to use and very stable. Calibration and maintenance is quick and simple."
Lloyd Gruginski
Chehalis WTP - Chehalis, WA
"The Pi products provide excellent value for money and represent the best municipal drinking water analyzers available."
John Clark
Chemtrac - Atlanta
“Servicing customers is much more than just solving problems or addressing complaints and Pi does that very competently with technical and quick efforts providing a good experience."
Clovis Tuchapski
Buckman - Latin America
"Pi's technical team has enabled us to be one step ahead of our competitors by adding value to our projects thanks to their fast and excellent support from the moment you first reach out.
Ibrahim Kaplin
Thermomed - Turkey
"Going from ORP control to amperometric chlorine sensor control has undoubtedly improved the pool water immensely!"
Chris Tedeschi
Link Automation - USA
"The Streaming Current Monitor from Pi is the best SCM I have ever used. The analyser responds quickly and has many powerful functions, which helps me save a lot of money."
Ye Yancong
Xiamen Xishan Water Plant - China
“Process Instruments has a broad range of high quality and user-friendly solutions for water-industry problems. The short lead times and great customer support make Pi a reliable partner.”
Péter Szabó
SC KATALIN NOHSE CHIMIST IMPORT SRL - Romania
"Process Instruments UK always have a high level of customer service. All our interactions with Pi have exceeded our expectations. It is always a pleasure working with you.”
Iñaki Seisdedos Rodríguez
Izasa Scientific - Spain
"The support from Pi and its partners is superb. They go above and beyond to ensure that, not only is their equipment perfect but that the process is working great too. Five Stars!"
Anthony Glitto
Equip Solutions - Illinois, USA
"Process Instruments UK always have a high level of customer service. All our interactions with Pi have exceeded our expectations. It is always a pleasure working with you.”
Rudi Tuffek
Allpronix - South Africa












